From both big pharma and more specialized drug repositioning companies presenting at the Drug Repositioning Conference in San Francisco last week, it became clear that if one can get an IP hook, the strategy allows one to quickly create significant monetizable value with a relatively small investment.
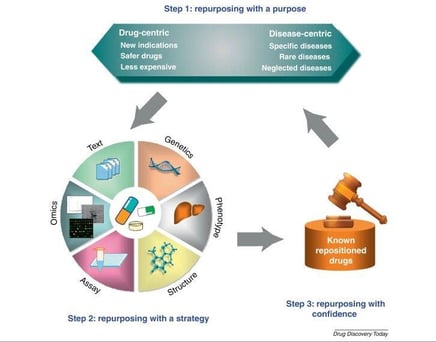
from: http://dx.doi.org/10.1016/j.drudis.2012.08.005
To highlight two memorable examples:
- Cephalon bought bdc for $70m with just an “animal use patent” for sciatica to compete w/ Amgen’s Embrel
- Adolor repositioned the potential anti-depressant Org4428 that stalled at phase 3 for depression as a non-opioid for pain, received a fast-track use patent, and successfully partnered the program with Organon (which was acquired by Schering-Plough, now Merck).
Bottom line is if you can show differentiation, capture IP, and show a cost-benefit commercial path to market, then repurposing is a fast (perhaps the fastest) route to an approved drug.
Barry Bunin chaired a webinar panel with Chris Lipinski, David Cavalla and Noel Southall. Download the talks below. We’ll provide full talk transcripts and archive these materials on the CDD Resources page for easy reference.
- David Cavalla slides and transcript
- Barry Bunin talk, slides and transcript
- Chris Lipinski talk, slides and transcript
- Noel Southall talk, slides and transcript
Tag(s):
Events
Other posts you might be interested in
View All Posts
Webinars
1 min
October 23, 2025
Augmented Design: Practical Applications of LLMs and Agentic Workflows in Medicinal Chemistry
Read More
Webinars
1 min
October 23, 2025
Distributed Operations: Building Competitive Advantage in Biotech Through Strategic Outsourcing
Read More
Webinars
1 min
October 23, 2025
OpenADMET Blind Challenge
Read More


